Fowler's Toad
Anaxyrus fowleri
Common Name: |
|
Scientific Name: |
Anaxyrus fowleri |
Etymology: |
|
Genus: |
Anaxyrus is Greek meaning "A king or chief" |
Species: |
fowleri is to honor S.P. Fowler, a naturalist from Massachusetts. |
Average Length: |
2 - 3 in. (5.1 - 7.5 cm) |
Virginia Record Length: |
|
Record length: |
3.75 in. (9.5 cm) |
Physical Description - This species is 2 to 3 inches (50-75 mm) long. Its dorsum color ranges from brown to olive to gray with a light middorsal stripe. The dark dorsal spots typically each have 3 or more warts. The cranial crests are small and connected to the parotoid glands. The venter is generally unspotted though some specimens have a large dark breast patch. Males are smaller than the females and have a black throat. This species hybridizes with Anaxyrus americanus americanus and Anaxyrus terrestris making identification sometimes difficult.

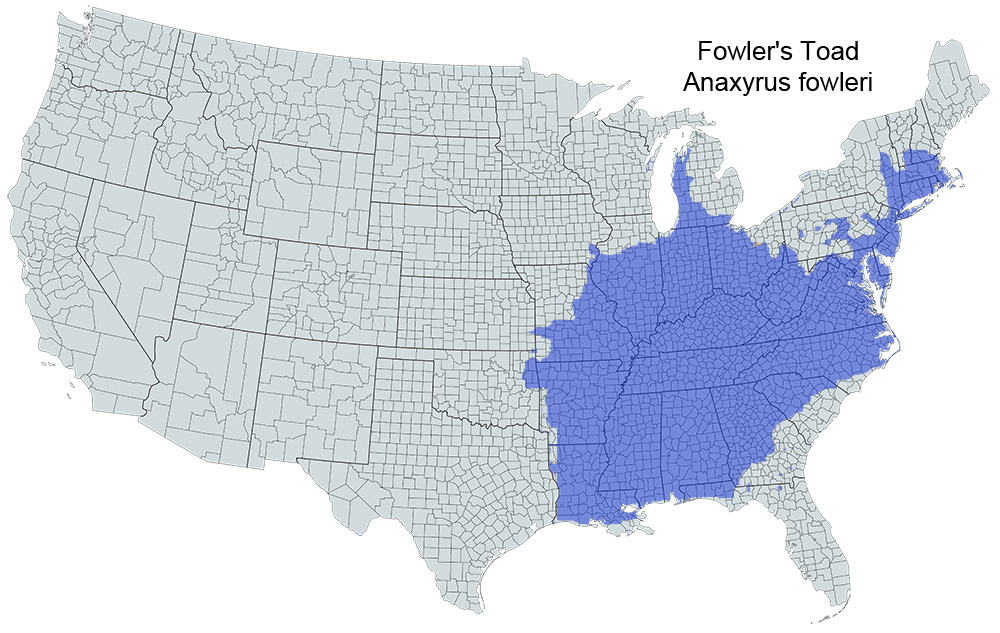
Historical versus Current Distribution - Fowler's Toads (Anaxyrus fowleri) occur throughout the eastern United States, exclusive of the southern Atlantic Coastal Plain and the Florida Peninsula (Burt, 1932; Wright and Wright, 1949; Conant and Collins, 1998). Their northern range edge is along an irregular line reaching from southeastern Iowa through northern Illinois and turning north to the south shore of Lake Michigan (Bailey, 1944; Smith, 1947, 1961; Minton, 1972; Harding, 1997) to Chicago (Higginbotham, 1939; Edgren and Stille, 1948). They reach their northern limit on the west side of Michigan’s Lower Peninsula, reaching almost to the Mackinac Strait in the north (Smith, 1961; Harding, 1997). The northern limit also encompasses Lake Erie in Ohio (Walker, 1946; Kraus and Schuett, 1982) and extends across northern Pennsylvania, New Jersey, southeast New York and southern Vermont, through southern New Hampshire to reach the Atlantic Coast almost to Maine (Stewart and Rossi, 1981; Kraus and Schuett, 1982; Shaffer, 1991; Harding, 1997).
At their eastern limit, A. Fowler’s Toads occur along the Atlantic coast from New Hampshire to southern North Carolina, including Massachusetts, Connecticut, New York, Delaware, Maryland, New Jersey, and Virginia (Burt, 1932; Babbitt, 1937; Latham, 1971a,b; Lazell, 1976; Miller, 1979; Klemens, 1993; Given, 1999). A. Fowler’s Toads occur on many nearshore islands along this stretch of the Atlantic coast including Cape Cod and its adjacent islands, Long Island, and islands off the Virginia coast (Harper, 1935; Latham, 1971a,b; Lazell, 1976; Hranitz at al., 1993). The range skirts the Coastal Plain from southern North Carolina across the middle of South Carolina and Georgia to the panhandle of Florida at the Gulf of Mexico (Miller, 1979; Martof et al., 1980; Ashton and Ashton, 1988; Hunter et al., 1992). A. Fowler’s Toads range across the Gulf Coast from there to the Florida Parishes of southeast Louisiana (Anderson et al., 1952; Liner, 1954; Mount, 1975; Ashton and Ashton, 1988; Dundee and Rossman, 1989).
The western edge of the range of A. Fowler’s Toads is ill defined, according to most sources, due to their intergradation with the more westerly Woodhouse’s toads (Anaxyrus woodhousii; Meacham, 1962; Johnson, 1987; Black and Sievert, 1989; Dundee and Rossman, 1989). Conant and Collins (1998) indicate a wide overlap of the two forms encompassing eastern Oklahoma, eastern Texas, western Arkansas, and much of Louisiana. The two toads’ interrelationship is especially complicated in the region of east Texas and western Louisiana where there exists a confusion of difficult-to-identify toads with intermediate morphologies. Bragg and Sanders (1951) named these animals Anaxyrus woodhousii velatus (or simply A. velatus according to Sanders [1987]; East Texas toads). The status of A. w. velatus is uncertain (see A. woodhousii account, this volume). Conant and Collins (1991), Sullivan et al. (1996a), and Dundee and Rossman (1989) view them as a variant of Woodhouse's toads. Dundee and Rossman (1989) also question the existence of this hybridization zone in Missouri and Oklahoma. The western edge of the range of unequivocal A. Fowler’s Toads runs from southeast Louisiana (Dundee and Rossman, 1989) through most of Arkansas almost to the Oklahoma border, and north across central Missouri to southeast Iowa, avoiding Kansas (Conant and Collins, 1998; Smith and Johnson, 1999). Delimiting the distribution of Fowler's Toads in the southeastern portion of their range may also be complicated due to their tendency to hybridize with southern toads (Anaxyrus terrestris; L.E. Brown, 1970) and Gulf Coast toads (Anaxyrus valliceps [now considered to be Coastal-Plain toads, A. nebulifer; see Mulcahy and Mendelson, 2000; Mendelson, this volume]; Fox et al., 1961; Wittliff, 1964).
The large range of A. Fowler’s Toads was neither fully appreciated nor well mapped until about 50 yr ago (Wright and Wright, 1949). In the late 1800s and for many years subsequent to the description of the species, the range was considered to be confined merely to the vicinity of the type locality in Massachusetts, as reported by Dickerson (1906). This is truly remarkable, as A. Fowler’s Toads are exceedingly common all through the Atlantic states and across their presently known range. At last, Allard (1907, 1908) reported what is now obvious: A. Fowler’s Toads occur commonly along the eastern seaboard as far south as at least northern Georgia. Ruthven (1917) and then Hubbs (1918) reported them in Michigan, Indiana, and Illinois, and then the true extent of their range rapidly began to be filled in. Compared to more recent maps in Conant and Collins (1998) and Conant (1958a, 1975), Wright and Wright (1949) did not register A. Fowler’s Toads as occurring along the Gulf of Mexico coast except in Louisiana, considered the range to encompass Louisiana and east Texas, and missed much of their occurrence in Arkansas, Pennsylvania, and along the coast of North Carolina.
The early nomenclatural history of A. Fowler’s Toads, reviewed by Kluge (1983), Sanders (1987), and Green (1989), is confused. According to Dexter (1973), naturalist S.P. Fowler (1862), at a meeting of the Essex Institute on 30 June 1858, reported the existence of a new and “hitherto undescribed” species of toad collected from Danvers, Massachusetts, and F.W. Putnam dubbed it “Anaxyrus fowleri,” a name printed in the Proceedings of the Essex Institute without description. Authorship of the species from that time until 1921 was generally ascribed to Putnam (e.g., Allen, 1868; Garman, 1884; Jordan, 1888; Cope, 1889; Allard, 1907, 1908) yet there existed no published description by Putnam, only his 1863 intent to publish (Dexter, 1966), which finally saw print in an article by Dexter (1973). Garman (1884) gave a brief description of Anaxyrus fowleri Putnam and, with the realization that Putnam’s intended description had not been published, Anaxyrus fowleri Garman was recognized. However, Myers (1931b), in reviewing the problem, concluded that Hinckley’s (1882) description of the tadpole as distinct from the tadpole of the American toad (Anaxyrus americanus) was the first actual description of the species and therefore had taxonomic priority over Garman’s (1884) account according to the rules of zoological nomenclature. The attribution to Hinckley has been accepted ever since. Following Smith (1934), Woodhouse's toads and Fowler's Toads had been considered conspecific as subspecies of Anaxyrus woodhousii (also spelled woodhousei) by most, but not all, authorities. Frost (1975), too, listed fowleri as a junior synonym of Anaxyrus woodhousii but acknowledged that this taxonomy was controversial. Smith (1934), in his consideration of the amphibians of Kansas, including the toads, concluded that both Woodhouse's toads and Fowler's Toads were equally different, and different in equal ways from American toads. He stated that their similarities were striking, citing egg membranes, song, cranial crests, and the spiny-ness of the skin. Differences he noted were size, clutch size, and color pattern. It is now evident, however, that A. Fowler’s Toads do not occur in Kansas (Collins, 1974, 1982). Burt (1935) considered woodhousii and fowleri to be conspecific, as he could distinguish no significant diagnostic differences between toads from the southeast and toads from the middle west, both of which may have been fowleri. Meacham (1962) added apparent weight to Smith’s (1934) taxonomy with an analysis of morphological characters of toads in east Texas, where A. Fowler’s Toads likewise do not occur. Meacham concluded that there was a zone of intergradation between Woodhouse's toads on the west and Fowler's Toads on the east and therefore, under a biological species concept, they were one species. Meacham (1962) used six head characters, largely from the cranial crests, in his analysis, but the characters he used are not independently variable (Green, 1989), invalidating many of the results. Sullivan et al. (1996a) summarized this and other evidence from behavior, morphology, and genetics with a conclusion (followed by Collins [1997]) that supported the recognition of these taxa as separate species.
Sanders (1987), in an idiosyncratic analysis of skull characters principally concerning the cranial crests, concluded that only A. Fowler’s Toads from Danvers, Massachusetts, and vicinity were truly Anaxyrus fowleri. According to Sanders, these toads had more closely spaced interorbital cranial crests than did A. Fowler’s Toads from elsewhere in the range, which he dubbed Anaxyrus hobarti. This taxonomic arrangement has resisted general acceptance.
Historical versus Current Abundance - A. Fowler’s Toads have been noted by numerous authors to be extremely abundant throughout much of their range (Allard, 1908; Martof, 1962a; Lazell, 1976; Klemens, 1993; Zampella and Bunnell, 2000), but especially in the northeast of their distribution. In the extreme south of their range, however, they are replaced by other species as the most common toad (Mount, 1975; Dundee and Rossman, 1989). A. Fowler’s Toads are more tolerant and dependent upon higher temperatures than are American toads, coincident with their generally more southerly distribution (Frost and Martin, 1971).
Historical abundances are unknown, but populations can vary widely in size in different years and at different places and, at times, may consist of large numbers of individuals (Breden, 1988; Green, 1992, 1997a; Hranitz et al., 1993). Lazell (1976) notes that A. Fowler’s Toads, with their penchant for dry scrub and open country, probably benefited from land clearing during the early days of European settlement in eastern North America. A. Fowler’s Toads once occurred virtually everywhere on Cape Cod and adjacent islands. Recently, however, Lazell (1976) reports that they were extirpated from Nantucket, Muskeget, Cuttyhunk, and, probably, Tuckernut islands between 1940 and 1960, which Lazell attributes to indiscriminate use of pesticides, notably DDT (See "Conservation" below).
Breeding - Reproduction is aquatic.
Breeding migrations - Fowler's Toads will converge at breeding ponds in late spring (Minton, 1972; Grogan and D. Bystrak, 1973; Laurin and Green, 1990), usually from one to a few weeks after sympatric American toads (Hoopes, 1930; Babbitt, 1937). In Florida, A. Fowler’s Toads breed from February–April (Ashton and Ashton, 1988); in Alabama, breeding may commence in March or April, although the peak of breeding activity is usually mid May (Mount, 1975). Toads may begin to breed in March in Louisiana but more commonly not until April (Dundee and Rossman, 1989). A. Fowler’s Toads breed in March–May in South Carolina and April–July in Virginia (Martof et al., 1980); April in West Virginia (Green and Pauley, 1987) and Tennessee (Huheey and Stupka, 1967); late April to July with its peak in mid May in Kentucky (Barbour, 1971); mid May to mid June in Michigan (Harding and Holman, 1992); mid May to June in Connecticut (Clarke, 1974a); late April to late June in Illinois (Smith, 1961); and late April to early July in the Great Lakes region (Harding, 1997). Breden (1988) recorded choruses beginning from 4–11 May at a minimum air temperature of 10 ˚C at Indiana Dunes on Lake Michigan. Also, at the toads’ northern range limit on Lake Erie, Green (1997a; unpublished data) has recorded A. Fowler’s Toads beginning to sing any time from the last days of April–24 May, at a minimum body temperature of 14 ˚C (Green, 1997; Blaustein et al., 2001; unpublished data). In Georgia, however, A. Fowler’s Toads do not call at air temperatures below 19 ˚C (Martof, 1962a). Many authors have documented the male-biased sex ratios of breeding aggregations of A. Fowler’s Toads (Aronson, 1944a; Fairchild, 1981; Breden, 1988; Hranitz et al., 1989; Laurin and Green, 1990). Breden (1988) found the ratio of females to total number of toads present to range from 0–0.6 but averaging 0.13 and 0.10 in two different years. However, this represents differences in behavior of the two sexes, as the population sex ratio did not differ significantly from 1:1, a finding echoed by Green (1997a).
Descriptions of the call of the male Fowler’s toad have been given by many authors (e.g., Harper, 1928; Wright and Wright, 1949; Bogert, 1960; Zweifel, 1968a; Green, 1982; Given, 1996; Sullivan et al., 1996a). Garman (1892) described it as a “prolonged and rather shrill scream,” although both he and Allen (1899) erroneously attributed the source to American toads late in their breeding season without suspecting that the sound came from a different kind of toad. At last tracing it to its true originators, H.A. Allard variously likened the call of Fowler's Toads to an “almost agonized wail” (Allard, 1907), a “penetrating droning scream” (Allard, 1908), or a “weird, wailing scream” (Allard, 1916). It usually sounds rather like a sort of muffled scream from a small and distressed sheep. Structurally, the call has the same characteristics as other Anaxyrus, including American toads, which make trilled calls, but the pulse frequency is so high that individual pulses cannot be discerned (Zweifel, 1968a; Green, 1982). The vocal sac of the male radiates the sounds more to the front of the animal than behind it (Gerhardt, 1975). The males in a chorus respond to each others' vocalizations and females orient to particular males’ calls based on persistence and intensity (Given, 1993, 1996). Not all males in a breeding aggregation will call, however, thus the intensity of chorus is not a good indicator of the actual number of animals present (Shirose et al., 1997). Males issue a grumbling, vibrating release call when handled, whether by humans or by other toads (Brown and Littlejohn, 1972; Leary, 2001a,b).
Breeding habitat - Fowler's Toads breed in the shallow water of permanent ponds, flooded low ground, temporary pools, farm ponds, roadside ditches, quiet streams, lake shores, or along the shallows of rivers (Wright and Wright, 1949; Smith, 1961; Mount, 1975; Collins and Wilbur, 1979; Green and Pauley, 1987; Breden, 1988; Dundee and Rossman, 1989). Breden (1988) described breeding ponds used by the toads as shallow with sandy bottoms and gradually sloping banks, vegetated primarily with sedges and bulrushes.
Egg deposition sites - Same as breeding habitat. Eggs are laid in long twin strands and are 3–4 mm in diameter (Wright and Wright, 1949).
Clutch size - Ovarian eggs are reported as 2,000–4,000 eggs by Birge et al. (2000), but clutch sizes have been reported as up to 7,000 (Martof et al., 1980), 8,000 (Wright and Wright, 1949; Barbour, 1971; Green and Pauley, 1987; Ashton and Ashton, 1988), or even 10,000 (Mount, 1975) eggs. The average clutch size, based on data from Clarke (1974b) from Connecticut, was 5,221 eggs/clutch. However, based on direct counts of eggs laid, clutch sizes ranging from 2,000–6,300, an average around 3,700, were found (unpublished data). Eggs hatch about 1 wk after laying (Smith, 1961; Martof et al., 1980).
Altig & McDiarmid 2015 - Classification and Description:
- Eastern Linear
- Arrangement 3 - Eggs oviposited as strings.
- Sub-arrangement B - Jelly tube unilayered and obvious; string long. Most of area except southeastern Coastal Plain and Florida and north of northern Ohio; ephemeral, nonflowing water; Ovum Diameter 1.0-1.4 mm; tube diameter 2.6-4.6 mm; tube with straight margins; partitions absent between uniserial or staggered eggs.
- Arrangement 3 - Eggs oviposited as strings.
Larvae/Metamorphosis - Larvae are black overall, except for the pale venter of the tail (Hinckley, 1882; Altig, 1970; Altig et al., 1998). Larvae are often observed in large aggregations (Breden et al., 1982) in stream and pond habitats, typically in standing water and resting on a muddy or sandy substrate. Details on larval development can be found in Bragg (1940b). Gosner and Black (1957b) found A. Fowler’s Toads to use ponds with pH between 5.5 and 6.6 in the New Jersey Pine Barrens, avoiding sphagnaceous ponds with lower pHs.
Tadpoles:
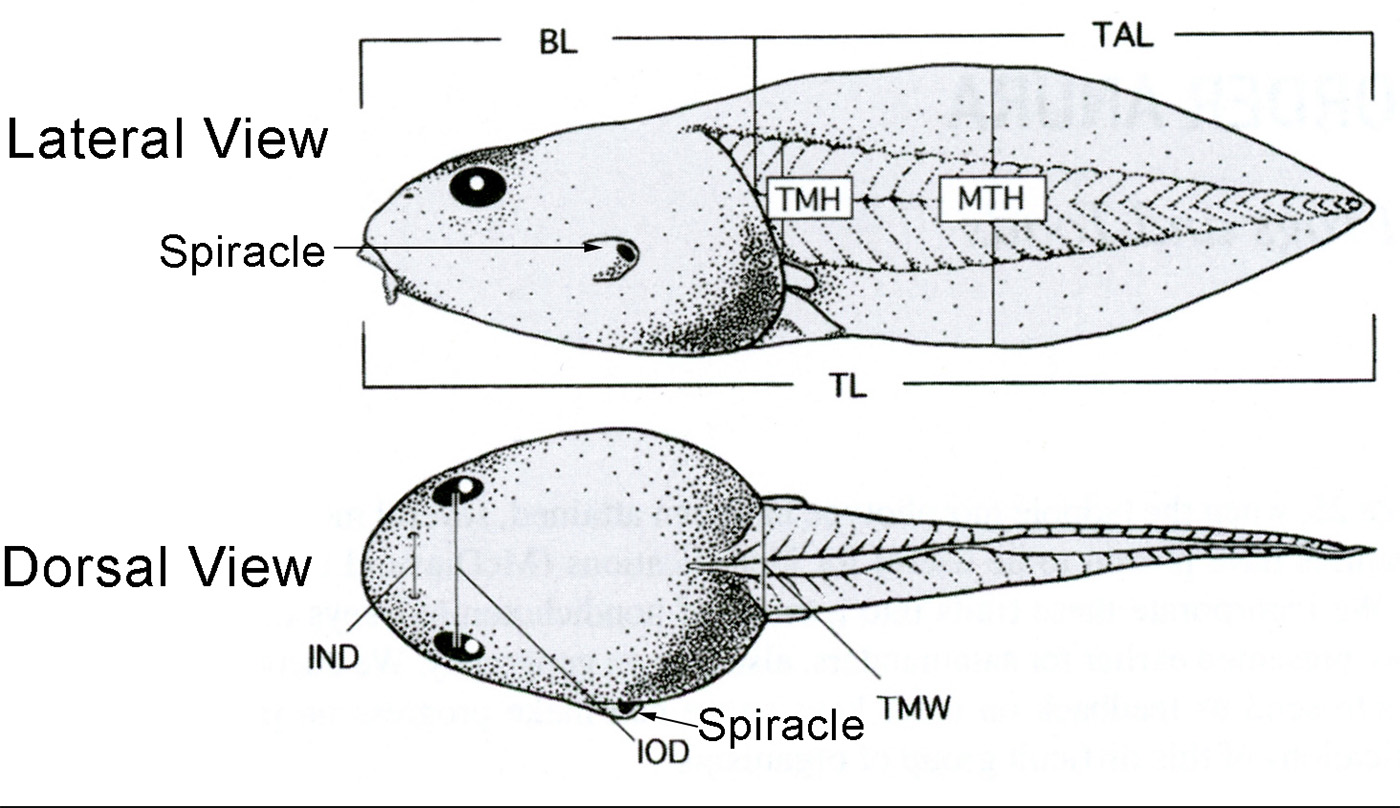
| Lateral View | Dorsal View |
|---|---|
| BL = Body Length | IND = Internarial Distance |
| MTH = Maximum Tail Height | IOD = Interorbital Distance |
| TAL = Tail Length | TMW = Tail Muscle Width |
| TL = Total Length | |
| TMH = Tail Muscle Height |
Length of larval stage - The larval period takes 40–60 d (Wright and Wright, 1949; Ashton and Ashton, 1988), and tadpoles transform at about 8–12 mm SVL (Wright and Wright, 1949; Martof et al., 1980; Breden, 1988). Fowler’s toad larvae tend not to do well in artificial ponds with higher pH and floating vegetation (Bunnell and Zampella, 1999).
Larvae Food - Larvae are suspension feeders and will take a variety of organic and inorganic material. b. Cover. Fowler’s toad tadpoles are active and benthic in behavior compared to co-occuring other species of tadpoles in the New Jersey Pine Barrens, and they decrease their activity in the presence of eastern newts (Notophthalmus viridescens) and black-banded sunfish (Enneacanthus obesus) even though the fish find them unpalatable (Lawler, 1989).
Larval polymorphisms Do not occur.
Features of metamorphosis - Metamorphosis takes place from late June to July in Illinois (Smith, 1961), mid June to August in New York (Wright and Wright, 1949), and late July to August in Kentucky (Barbour, 1971). Newly metamorphosed animals tend to average smaller in size under increasingly crowded conditions, with concomitant reduction in their initial stamina and jumping ability (John-Alder and Morn, 1990).
Post-metamorphic migration - From wetland breeding sites to upland feeding sites.
Juvenile Habitat - Similar to habitat characteristics of adults.
Adult Habitat - Fowler's Toads occur in areas with loose, well-drained gravelly or sandy soils, including sand dunes, sandy deciduous woodland, and rocky, poorly vegetated areas (Hubbs, 1918; Smith, 1961; Minton, 1972; R.L. Brown, 1974; Klemens, 1993). Wright and Wright (1949) note that Fowler's Toads can be common along roadsides, near homes, and in fields, pastures, gardens, and sand dunes. They are a typical species of the New Jersey Pine Barrens (Zampella and Bunnell, 2000). Lazell states that whereas American toads on Cape Cod occur in wet deciduous woodlands and uplands, A. Fowler’s Toads prefer dry scrub, sand dunes, and open country. Klemens (1993) likewise observes in Connecticut that American toads are located in moist shady woodland but A. Fowler’s Toads are to be found on dry sunny rock ledges. In Louisiana, where A. Fowler’s Toads co-occur with southern toads (Anaxyrus terrestris), A. Fowler’s Toads are in the bottomlands whereas the southern toads occupy higher ground (Dundee and Rossman, 1989). Huheey and Stupka (1967) observe that Fowler's Toads occur up to 1,200 m elevation in the Great Smoky Mountains of Tennessee but are much more common at lower elevations. Bossert et al. (2003) report that Fowler's Toads will use northern diamond-backed terrapin (Malaclemys terrapin terrapin) borrows as refugia.
Home Range Size - A. Fowler’s Toads will establish small home ranges, along the shorelines of lakes or large ponds. On Lake Michigan, Stille (1952) recorded them emerging from under the sand about 60–210 m from the water’s edge and moving to the beach to rehydrate and forage over about 8 m of shorefront. Tracking three different individuals, Clarke (1974a) calculated their home range minimum polygons to be 2,742 m2, 2,398 m2, and 526 m2, respectively. Adult A. Fowler’s Toads will return to their home ranges when displaced up to 1.28 km (R.J. Nichol, quoted in Oliver, 1955a) and will occupy the same home range year after year (Clarke, 1974a). They apparently can orient themselves using olfactory cues and a sun compass (Landreth and Ferguson, 1968; Grubb, 1973a).
Territories - A. Fowler’s Toads are not territorial in any way.
Aestivation/Avoiding Dessication - Fowler's Toads escape hot and dry conditions by burrowing into the ground (Harding and Holman, 1992) or finding burrows (Bossert et al., 2003) and become less active at temperatures above about 25 ˚C (Hadfield, 1966).
Seasonal Migrations - After the breeding season, adult A. Fowler’s Toads tend to move to beaches alongside larger water bodies to take up their “summer quarters” (Breden, 1988). This movement pattern has been observed at Indiana Dunes on Lake Michigan by Shelford (1913), Hubbs (1918), Stille (1952), and Breden (1982), and at Long Point on Lake Erie, Canada, by Green (1997a). They are later joined on the beach by the young of the year after metamorphosis (Breden, 1988). Non-breeding juveniles tend to remain in “summer quarters” throughout their active season. Juveniles tend to disperse more widely than adults (Clarke, 1974b; Breden, 1988). Fowler's Toads are generally nocturnal (Higginbotham, 1939; Clarke, 1974a), but can be seen during the day in humid, overcast weather (Smith, 1961; Green and Pauley, 1987).
Torpor (Hibernation) - In Connecticut, Fowler's Toads are dormant for 7 mo of the year (Clarke, 1974a). They burrow into the sand or soil during the winter (Harding and Holman, 1992) to a depth of about 15–30 cm by late winter (R. Latham quoted in Oliver, 1955a). They may also overwinter in burrows (Bossert et al., 2003).
Interspecific Associations/Exclusions - Wright and Wright (1949) note that wherever Fowler's Toads are sympatric with American toads, Fowler's Toads occur in rivers, streams, or lake beaches. The two species tend to have different temperature tolerances (Frost and Martin, 1971) and habitat preferences: American toads in forests, A. Fowler’s Toads in more open sandy areas and savannas (Smith, 1961; Mount, 1975; Green and Pauley, 1987; Johnson, 1987). Although both Fowler's Toads and American toads are found throughout the Midwest, where one species commonly is found the other is typically rare (Minton, 1972). Compared to American toads, A. Fowler’s Toads breed later and at warmer temperatures (Barbour, 1971; Green, 1982). Nevertheless, overlaps in breeding season do occur (Green, 1984). There had been long-standing confusion between the two species in the literature until their identities were clearly defined by Myers (1927, 1931b) and Netting (1930) and explained by Wright and Wright (1949). Dickerson (1906), for instance, includes a photograph labeled American toads although the animals portrayed are clearly A. Fowler’s Toads. Usually unmentioned by accounts of characters that differentiate Fowler's Toads from American toads is the distinctive smell of Fowler's Toads, which is reminiscent of the smell of unroasted peanuts or, according to Miller and Chapin (1910), Ailanthus sp. (Tree of Heaven) wood.
A. Fowler’s Toads, as with all the members of the Anaxyrus americanus group of toads (Blair, 1959, 1963a, 1972a; Guttman, 1969; Martin, 1973) are notorious hybridizers (A.P. Blair, 1941; W.F. Blair, 1964a, 1972b; Green, 1984, 1996; Sanders, 1987; Green and Parent, 2003). A. Fowler’s Toads are broadly sympatric with American toads and hybridize with them in numerous scattered localities (Allard, 1908; Miller and Chapin, 1910; Hubbs, 1918; Myers, 1927; Pickens, 1927a; Blair, 1941; Volpe, 1952, 1955b; Cory and Manion, 1955; Zweifel, 1968a; L.E. Brown, 1970; Jones, 1973; Green, 1982, 1984; Green and Parent, 2003). They also have been known to hybridize with Coast-Plain toads (Fox et al., 1961; Wittliff, 1964) and Anaxyrus terrestris (L.E. Brown, 1970). Furthermore, diagnostic characters, such as the extent of ventral spotting, are not necessarily consistent from place to place (Blair, 1943), and newly metamorphosed animals may not have their distinguishing features fully developed, which continues to engender confusion and misidentification. On the basis of mitochondrial DNA sequences, Masta et al. (2002) discerned three distinct mitochrondrial phylogroups within what is currently known as Fowler’s toad.
Age/Size at Reproductive Maturity - Post-metamorphic growth rate is rapid (Labanick, 1976a; Claussen and Layne, 1983). In Connecticut, Clarke (1974b) recounts an average 6.58-fold increase in length during the first year, whereas Labanick (1976a) calculated an even faster rate of growth, averaging 0.36 mm/d among post-metamorphic toads in Indiana from mid June to mid August. Females grow faster than males to reach larger size (Clarke, 1974b), and both males and females usually reach reproductive maturity at 2 yr of age (Breden, 1987). Particularly rapidly growing individuals may reach maturity within 1 yr of metamorphosis (Kellner and Green, 1994). Breeding males range from 42–74.5 mm, females from 55–82 mm SVL (Wright and Wright, 1949). Martof et al. (1980) say that adult A. Fowler’s Toads range in size from 50–82 mm in the Carolinas. Mount (1975) recounts a maximum SVL of 85 mm in Alabama. Dundee and Rossman (1989) say that the toads range from 51–76 mm in Louisiana. Green and Pauley (1987) give a size range of 51–84 mm for toads in West Virginia. Breden (1988) found adult males to average 58.8 mm ± 0.09 (s.e.) and adult females to average 62.2 mm ± 0.27 (s.e.) in northern Indiana. The average size of A. Fowler’s Toads tends to be negatively correlated with their abundance (Hranitz et al., 1993; Green, 1997a).
Longevity - A. Fowler’s Toads live a maximum 5 yr in the wild (Kellner and Green, 1995). An adult Fowler’s toad survived at the Philadelphia Zoo for 2 yr, 5 mo, and 3 d (Bowler, 1977). Survivorship is low. Clarke (1972) concluded that toe-clipping of toads in order to study them lowered their survivorship, and Parris and McCarthy (2001) found that the effect was correlated with the number of toes removed. In any case, the survivorship rate is very low. The curve is Type III and decidedly J-shaped (Breden, 1988). Clarke (1977) calculated about 22.5% annual survival among post-metamorphic toads in Connecticut.
Feeding Behavior - A. Fowler’s Toads feed on a variety of invertebrates, especially beetles and ants (Metcalf, 1921; Bush, 1959; Bush and Melnick, 1962; Klimstra and Myers, 1965; Latham, 1968; R.L. Brown, 1974; Clarke, 1974c; Labanick and Schleuter, 1976; Gould and Massey, 1984). Cope (1889) states that they feed readily on flies but will not eat earthworms. Smith (1961) reiterates this aversion to earthworms, mentioning that captives are reluctant to eat them. Post-metamorphic toadlets unsurprisingly consume smaller prey than adults, largely collembolans, aphids, and fly larvae (Clarke, 1974c). Although A. Fowler’s Toads routinely hop, especially when disturbed, they approach prey by walking (Heatwole and Heatwole, 1968).
Predators - Eastern Hog-nosed Snakes (Heterodon platyrhinos) appear to be immune to Fowler's Toad toxins and thus can and will feed on them (Surface, 1906; Edgren, 1955; Lazell, 1976; Shaffer, 1991). Some birds, such as shrikes (Laniidae) and bitterns (Ardeidae), have been known to take A. Fowler’s Toads (Latham, 1970, 1971c). Clarke (1977) notes that American Bullfrogs (Lithobates catesbeiana) and raccoons (Procyon lotor) will eat A. Fowler’s Toads.
Anti-Predatory Mechanisms - A toad’s noxious skin secretions afford protection against some predators (Kruse and Stone, 1984; Harding and Holman, 1992). Clarke (1977) recounts that sometimes A. Fowler’s Toads may be found with the scars of mammal bites or bird pecks from unsuccessful attempts to make them food. Fowler's Toads also rely upon their general resemblance to sand and dirt, coupled with immobility (Dodd, 1977a; Harding and Holman, 1992) to escape detection. A Fowler’s toad’s normal locomotor gait, which consists of both walking and hopping, switches entirely to a more energetically efficient hopping when the toad seeks to escape after detection (Walton, 1988; Walton and Anderson, 1988; Anderson et al., 1991). Under duress, A. Fowler’s Toads can cover a distance of up to 37 cm/hop on sand, but generally manage about 13–15 cm/hop (Rand, 1952). Fowler's Toad tadpoles are active and benthic in behavior compared to other co-occuring species of tadpoles in the New Jersey Pine Barrens, and they decrease their activity in the presence of eastern newts (Notophthalmus viridescens) and black-banded sunfish (Enneacanthus obesus) even though the fish find them unpalatable (Lawler, 1989).
Diseases - Infection by Mycobacterium is known (Shiveley et al., 1981). R. Parasites. The nematode Spinitectus gracilis (Jilek and Wolff, 1978), among other endoparasitic helminths (Ashton and Rabalais, 1978; McAllister et al., 1989), plagues A. Fowler’s Toads. Unidentified cilate parasites have recently been described from Fowler's Toad tadpoles (Vences et al., 2003).
Conservation Lazell (1976) attributes the extirpation of A. Fowler’s Toads from Nantucket, Muskeget, Cuttyhunk, and, probably, Tuckernut islands between 1940 and 1960 to indiscriminate use of pesticides, notably DDT, which is one among many environmental contaminants to which A. Fowler’s Toads have been or continue to be exposed. Birge et al. (2000) consider A. Fowler’s Toads, compared to other amphibians, to be tolerant of organic contaminants, including for example carbon tetrachloride and chloroform, with a mean LC50 of 13.3 mg/L, but A. Fowler’s Toads are less tolerant of atrazine than expected. However, the organophosphate insecticide Azinphos-methyl (Guthion®) is the most toxic of many such chemicals for A. Fowler’s Toads, with LC50 measured at 0.13 mg/L (Sanders, 1970) or 0.109 mg/L (Mayer and Ellersieck, 1986). The organochlorides Endrin, Toxaphene, Dieldrin, Aldrin, DDT, and Lindane, in decreasing order, are also highly toxic to larval A. Fowler’s Toads (Sanders, 1970). The LC50 for hatchling Fowler’s toad tadpoles exposed to the organic contaminant Arochlor 1254 was 38.2 Fg/L (Birge et al., 1978). Four days post hatching, the tadpoles’ sensitivity increased to 3.7 Fg/L. Effects of the contaminant include lordosis, scoliosis, and abdominal edema. Adult A. Fowler’s Toads are also susceptible to organochloride poisoning (Ferguson and Gilbert, 1968) and exhibited accumulated residues of fenvalerate, a pyrethroid insecticide, after aerial spraying of cotton fields (Bennett et al., 1983).
In the presence of contaminating metals, according to Birge et al. (2000), A. Fowler’s Toads’ highest tolerances are for Cesium (LC50 = 1,076 mg/L) and Magnesium (LC50 = 807 mg/L) but they are least tolerant of Chromium (LC50 = 0.11 mg/L), Gallium (LC50 = 0.13 mg/L), Titanium (LC50 = 0.24 mg/L), or Aluminum (LC50 = 0.28 mg/L). A. Fowler’s Toads are considered more tolerant of Copper (LC50 = 25 mg/L; Linder and Grillitsch, 2000) and Zinc (LC50 = 87 mg/L; Birge et al., 2000) than are most other amphibians.
A. Fowler’s Toads also show effects of lowered pH due to acid precipitation. Juveniles suffer decreased growth rate when raised as larvae at their lowest tolerable pH (Freda and Dunson, 1986).
References for Life History
- Altig, Ronald & McDiarmid, Roy W. 2015. Handbook of Larval Amphibians of the United States and Canada. Cornell University Press, Ithaca, NY. 341 pages.
- AmphibiaWeb. 2020. University of California, Berkeley, CA, USA.
- Conant, Roger and, Collins, John T., 2016, Peterson Field Guide: Reptiles and Amphibians, Eastern and Central North America, 494 pgs., Houghton Mifflin Company., New York
- Duellman, William E. and, Trueb, Linda, 1986, Biology of Amphibians, 671 pgs., The Johns Hopkins University Press, Baltimore
- Martof, B.S., Palmer, W.M., Bailey, J.R., Harrison, III J.R., 1980, Amphibians and Reptiles of the Carolinas and Virginia, 264 pgs., UNC Press, Chapel Hill, NC
- Wilson, L.A., 1995, Land manager's guide to the amphibians and reptiles of the South, 360 pp. pgs., The Nature Conservancy, Southeastern Region, Chapel Hill, NC
Photos:
*Click on a thumbnail for a larger version.
Verified County/City Occurrence
Accomack
Albemarle
Alleghany
Amelia
Amherst
Arlington
Augusta
Bath
Bedford
Brunswick
Buckingham
Campbell
Caroline
Carroll
Charles City
Charlotte
Chesterfield
Clarke
Culpeper
Cumberland
Dinwiddie
Fairfax
Fauquier
Fluvanna
Frederick
Giles
Gloucester
Goochland
Grayson
Greene
Greensville
Halifax
Hanover
Henrico
Henry
Isle of Wight
James City
King and Queen
King George
King William
Lancaster
Lee
Loudoun
Louisa
Lunenburg
Madison
Mathews
Mecklenburg
Montgomery
Nelson
New Kent
Northampton
Northumberland
Nottoway
Orange
Page
Patrick
Pittsylvania
Powhatan
Prince Edward
Prince George
Prince William
Pulaski
Rappahannock
Richmond
Rockbridge
Russell
Scott
Shenandoah
Smyth
Southampton
Spotsylvania
Stafford
Surry
Sussex
Warren
Westmoreland
Wise
York
CITIES
Chesapeake
Fredericksburg
Hampton
Lynchburg
Newport News
Richmond
Suffolk
Virginia Beach
Williamsburg
Winchester
Verified in 79 counties and 10 cities.
U.S. Range