Jefferson Salamander
Ambystoma jeffersonianum
Common Name: |
Jefferson Salamander |
Scientific Name: |
Ambystoma jeffersonianum |
Etymology: |
|
Genus: |
Amby is Greek for "a cup", stoma is Greek for "a mouth" |
Species: |
jeffersonianum is in honor of Jefferson College in Canonsburg, PA and indirectly for the naturalist/President Thomas Jefferson. |
Average Length: |
4.3 - 7 in. (10.7 - 18 cm) |
Virginia Record Length: |
|
Record length: |
8.3 in. (21 cm) |
Virginia Wildlife Action Plan Rating Tier IV (Moderate Conservation Need) - The species may be rare in parts of its range, particularly on the periphery. Populations of these species have demonstrated a significant declining trend or one is suspected which, if continued, is likely to qualify this species for a higher tier in the foreseeable future. Long-term planning is necessary to stabilize or increase populations.
Physical Description - The body is comparatively long and moderately slender. Above, they are dark brown to almost black, with pale blue flecks on the lower sides. The old adults sometimes have the blue spots lacking. here are usually 12 costal grooves. The toes are long and slender. The length of this species is up to 185 mm. Mature males are large, long-legged, broad snouted, and light-colored. The females are gray to gray-brown with fine bluish flecks.
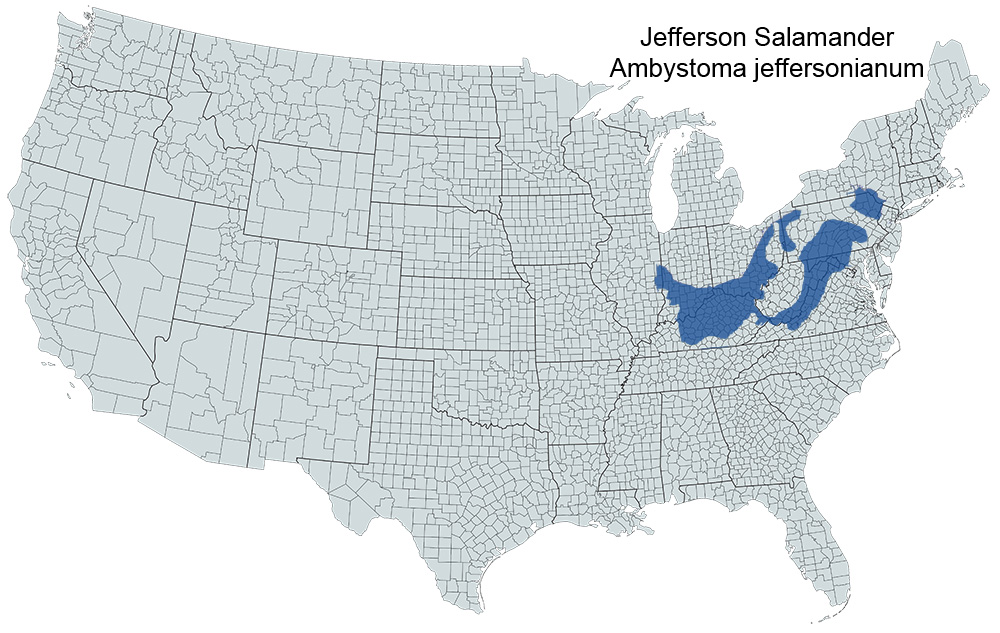
Historical versus Current Distribution - Because of the difficulty of distinguishing Jefferson Salamanders (Ambystoma jeffersonianum) from those of unisexual Ambystoma populations of hybrid origin, the status of Jefferson Salamanders is uncertain and their ecology poorly understood throughout much of their range. Early studies (prior to the mid 1960s) need to be viewed with caution because many may include blue-spotted Salamanders (A. laterale) or unisexual populations. Jefferson Salamanders are distributed in the United States from eastern Illinois and south-central Kentucky northeast to northern Virginia and southwestern New England (Petranka, 1998). There is no evidence to indicate that current distributions differ from historical distributions, but populations have been lost due to habitat destruction, alteration, and acidification (Sadinski and Dunson, 1992; Rowe and Dunson, 1993).
Historical versus Current Abundance - The relative abundance of Jefferson Salamanders is uncertain because they often coexist with unisexual Ambystoma hybrids. Recent studies indicate that unisexual populations have a larger range than previously thought (Rye et al., 1997) and often outnumber Jefferson Salamanders when they are syntopic (Uzzell, 1964; Nyman et al., 1988; Bogart and Klemens, 1997; unpublished data). Jefferson Salamanders are considered Locally Common–Rare in New England (DeGraaf and Rudis, 1983) and are on the Special Concern list in Vermont, Massachusetts, and Connecticut (Hunter et al., 1999). They are only known from two counties in eastern Illinois (Phillips, 1991) and have recently been listed as state Threatened. The relative abundance of Jefferson Salamanders in Indiana is considered occasional (Simon et al., 1992) or uncommon (unpublished data). Long-term studies from Indiana (S. Cortwright, personal communication) and Ohio (Brodman, 1995, 2002) indicate that some populations are stable. From 1989–'95 there were 36 new township records of Jefferson Salamanders in Ohio. In Pennsylvania, the total number of eggs that females deposit is positively correlated with pH levels and negatively correlated with aluminum levels (Rowe and Dunson, 1993; see also Petranka, 1998). At a pH of < 4.5, eggs and larvae will perish. Given that over half of the ponds studied in a region of Pennsylvania had a pH of ≤ 4.5, the abundance of Jefferson Salamanders is likely much lower than it was historically. Of the specimens examined from 106 sites in New York and New England, 70% are unisexual hybrids and most of the rest (23%) are blue-spotted Salamanders (Bogart and Klemens, 1997). Fewer than 7% of the specimens collected were Jefferson Salamanders and < 3% of the sites contain Jefferson Salamander populations that were not syntopic with unisexual populations (Bogart and Klemens, 1997). Jefferson Salamanders are common in the western panhandle of Maryland (Thompson et al., 1980; Thompson and Gates, 1982). Little is known about the relative abundance of southern populations in Kentucky, West Virginia, and Virginia.
Breeding - Reproduction is aquatic.
Breeding migrations - Jefferson Salamanders are among the first amphibians to breed and are the earliest Ambystoma species to breed, with the exception of the fall-breeding marbled Salamanders (Ambystoma opacum; Brodman, 1995; Petranka, 1998, Minton, 2001). Jefferson Salamanders breed as early as December–January in southern Indiana (P.K. Williams, 1973; Minton, 2001) and Kentucky (Smith, 1983) or as late as March in northern Ohio (Downs, 1989a; Brodman, 1995) and Pennsylvania (Mohr, 1931). They migrate from upland overwintering sites to wetland breeding sites. Males typically migrate first and will move while the ground remains frozen (Bishop, 1941a, 1943; Douglas, 1979; Douglas and Monroe, 1981; Downs, 1989a,b; Brodman, 1995; see Petranka, 1998). Warm nighttime rains or heavy snowmelts typically trigger spring breeding migrations; adults will migrate during the day under overcast skies (Bishop, 1941a). Adults breed early enough in the season that they can be caught out and killed by cold snaps (Petranka, 1998). Adults in southern populations occasionally will migrate to breeding wetlands in autumn, where they overwinter (Douglas and Monroe, 1981; Petranka, 1998).
Breeding in northern populations tends to occur in single bouts that usually last only a few days while southern populations breed in several bouts interrupted by cold weather (Bishop, 1941a; Douglas, 1979; Brodman, 1995). Sex ratios of males:females at any one time are usually greater than 3:1, with males staying for 16–30 d and females for 19–21 d (Collins, 1965; Downs, 1989b; Petranka, 1998). In some populations males breed annually while females skip ≥ 1 yr before returning to breed (P.K. Williams, 1973; Petranka, 1998). After breeding, adults migrate from wetlands back to underground retreats in the forest floor. Douglas and Monroe (1981) show that it takes about 45 d to move ≥ 250 m to these sites. Jefferson Salamanders have been found between 250–1,600 m from breeding sites (Bishop, 1941a; P.K. Williams, 1973; Downs, 1989b).
Jefferson Salamanders are one of four species that participate in the unisexual Ambystoma complex present in recently glaciated areas of the northeastern United States. In some ponds supporting this complex, unisexual female salamanders may outnumber males (Uzzell, 1964; Wilbur, 1971; Nyman et al., 1988; see also Petranka, 1998), and in these ponds Jefferson Salamander females will interfere with amplexing pairs (Bishop, 1941a). Jefferson Salamander males lay fewer spermatophores when courting females of the unisexual Ambystoma complex than when courting conspecific females (Petranka, 1998).
Breeding habitat - Adults typically breed in vernal and semi-permanent woodland pools but also occasionally in permanent, fishless woodland ponds (Bishop, 1941a; Douglas and Monroe, 1981; Brodman, 1995). Breeding ponds tend to be isolated from larger water bodies such as oxbows and lakes and tend to be cooler, more turbid and contain more aquatic vegetation than ponds that are not used (Thompson et al., 1980).
Egg deposition sites - Females begin laying eggs on submerged twigs, tree branches, and emergent vegetation 1–2 d following mating. Egg masses on firm supports such as twigs are ovoid; eggs laid on grasses and other vegetation are scattered. Females preferentially lay their eggs on the thin twigs of fallen branches and submerged ends of live willow branches (personal observations). Eggs are 2–2.5 mm in diameter. In time, many egg masses are colonized by symbiotic green algae Oophila sp. (Gatz, 1973). Average eggs/mass range from 14–31 (Smith, 1911a; Bishop, 1941a; Seibert and Brandon, 1960; Smith, 1983; Brodman, 1995).
Clutch size - Ovarian egg counts vary from 140–280 (Bishop, 1941a; Uzzell, 1964). Egg densities range from 57 eggs/m2 in Ohio (Brodman, 1995) to 123 eggs/m2 in Indiana (Cortwright, 1988) to 469 eggs/m2 in Kentucky (Douglas, 1979).
Altig & McDiarmid 2015 - Classification and Description:
- Eastern Mass - Oviposited as masses - Because capsular chamber present, centers of ova below center of inner jelly layer.
- Arrangement 2 - Mass diameter 45 mm or greater.
- Sub-arrangement A - Eggs deposited in temporary wooded pools; Ovum Diameter 2.0-2.5 mm; 3 jelly layers; jelly matrix watery.
- Arrangement 2 - Mass diameter 45 mm or greater.
Embryonic periods range from 3–14 wk, depending on temperature and latitude (Bishop, 1941a; Smith, 1983; Brodman, 1995). Embryonic survivorship tends to be high (71–96%) when the pH is ≥ 6.0 (Rowe and Dunson, 1993; Brodman, 1995).
Length of larval stage - From 2–4 mo and at sizes ranging from 52–78 mm (Bishop, 1941a; Minton, 1954; Downs, 1989b). Survival is reported to be < 1% in some populations (Thompson et al., 1980; Downs, 1989b), but can be higher in others (Pough and Wilson, 1977; Brodman, 1996; S. Cortwright, personal communication).
Larvae Food - Jefferson Salamander larvae are opportunistic and size-selective feeders that are gape-limited because they swallow most prey whole. Larvae take a variety of prey including ostracods, cladoceran and copepod zooplankton, nematodes, snails, and a range of aquatic and adult insects including chironomid (Diptera) larvae (Smith and Petranka, 1987; Petranka, 1998). Larvae are aggressive feeders and will cannibalize smaller larvae and prey upon spotted Salamander (Ambystoma maculatum) larvae (Brandon, 1961; Smith and Petranka, 1987; Brodman, 1996, 1999b). Smaller Jefferson Salamander larvae are sometimes found with missing limbs, gills, or tails (Petranka, 1998; personal observations).
Cover - Larvae are nocturnal, emerging from vegetation, where they are found during the day, to feed either on the wetland bottom or to float and feed in the water column. Nocturnal vertical migration and stratification has been observed in some, but not all, Jefferson Salamander populations (Anderson and Graham, 1967; Brodman, 1995). Jefferson Salamander larvae will use leaf litter and algae patches as refuges in the presence of predatory eastern tiger Salamander (Ambystoma tigrinum) or marbled Salamander larvae (Brodman and Jaskula, 2002).
Larval polymorphisms - While cannibalism is known, cannibal morphs (sensu Powers, 1907) have not been documented. Clanton (1934) and Bishop (1943) noted that there were dark and light forms in many populations of what was then thought to be Jefferson Salamanders. Minton (1954) clearly distinguished these forms as two species (Jefferson and blue-spotted Salamanders). Uzzell (1963, 1964) later found that the Jefferson Salamanders and blue-spotted Salamanders were distinct diploid species and that there were two sympatric all-female populations that were unisexual hybrids with triploid chromosome numbers. These unisexual Ambystoma populations typically use gynogenetic reproduction in which the unisexual females use male blue-spotted Salamanders, Jefferson Salamanders, small-mouth Salamanders, or tiger Salamanders to stimulate egg laying without the incorporation of male genes in the progeny (see the unisexual Ambystoma account by Phillips and Mui, this volume, for an explanation of this phenomenon). Many genomic combinations of unisexual hybrids are now known between blue-spotted Salamanders and Jefferson Salamanders (JLL, JJL, JL, JLLL, LLLL, and JLLLL [with initials denoting the species name of the parental genetic component as follows: J = jeffersonianum; L = laterale]), small-mouth Salamanders (LT, LLT, LTT, JLT, TTT, JJLT, LLLT, LLTT, and LTTT [T = texanum]), and tiger Salamanders (JLTi, LTTi, and LTTTi [Ti = tigrinum]). Because of this confusion, little research has been done on the autecology of Jefferson Salamanders. The historical literature can also be confusing. Clanton (1934) and Wacasey (1961) performed detailed ecological studies on "Jefferson Salamanders" in Michigan. However, the range of the Jefferson Salamanders does not extend into Michigan, so these widely cited studies describing Jefferson Salamander ecology must refer to either blue-spotted Salamanders or unisexual hybrids.
Features of metamorphosis - Jefferson Salamander larvae grow fast and can complete development in 2–3 mo. Metamorphosis usually occurs in late July to early August in northern Ohio, but can occur as early as June if the pond dries (personal observations). In southern Indiana, larvae have transformed as early as late May (Minton, 2001), and larvae have been found in ponds as late as November, indicating that larvae may overwinter in some populations (Cortwright, 1988).
Post-metamorphic migrations - Juveniles move a mean of 92 m (range 3–247 m) away from breeding wetlands into floodplain and upland forest floor habitats during their first 10 d (P.K. Williams, 1973).
Neoteny Not known to occur.
Juvenile Habitat - Little is known about the terrestrial ecology of Jefferson Salamanders (Petranka, 1998). In northern populations, juveniles are more active on the forest floor than are adults or juveniles in more southern populations (Green and Brant, 1966; Petranka, 1998). Juveniles appear to spend most of their time in burrows, where they feed on soil invertebrates (Petranka, 1998).
Adult Habitat Jefferson Salamanders are rarely caught above ground outside of breeding migrations. When encountered, they are typically scattered in deciduous forests near suitable breeding wetlands (Petranka, 1998). Adults live in burrows, including rodent burrows (P.K. Williams, 1973; Douglas and Monroe, 1981), and are found more often in well-drained upland forest sites than are other species of forest-dwelling Ambystoma (Downs, 1989b; Petranka, 1998). F. Home Range Size. Observations of post-breeding adults returning to burrows (Bishop, 1941a; P.K. Williams, 1973; Douglas and Monroe, 1981) over long distances (250–1,600 m) suggest that Jefferson Salamanders have home ranges that they retain from year to year.
Territories - Unknown.
Aestivation/Avoiding Dessication - Unknown, but individuals likely respond to dry conditions by going deeper into burrows.
Seasonal Migrations - Jefferson Salamanders migrate to their wetland breeding ponds from the underground burrows they use for overwintering (Douglas and Monroe, 1981). Following breeding, 3–4 wk later, they migrate back to feed and grow, often to their overwintering burrow (P.K. Williams, 1973; see also Faccio, 2003). Populations in south-central Kentucky have been observed in autumn migrations (Douglas and Monroe, 1981). Other migrations are unknown, although animals probably move nearer or farther from the soil surface with increasing or decreasing moisture conditions.
Torpor (Hibernation) - In burrows below the frost line.
Interspecific Associations/Exclusions - Jefferson Salamander populations frequently coexist and share breeding sites with unisexual Ambystoma hybrid Salamanders, marbled Salamanders, spotted Salamanders, small-mouthed Salamanders, eastern newts (Notophthalmus viridescens), wood frogs (Rana sylvatica), and spring peepers (Pseudacris crucifer; Thompson and Gates, 1982; Cortwright, 1988; Downs, 1989b; Sadinski and Dunson, 1992; Brodman, 1995; personal observations). They can share habitat with spotted Salamanders as often as 40% of the time in some parts of their range (Thompson and Gates, 1982). In regions of sympatry, Jefferson Salamanders and blue-spotted Salamanders are not known to share breeding sites (Anderson and Giacosie, 1967; Nyman et al., 1988; Bogart and Klemens, 1997). Studies on the interactions among Jefferson Salamanders, marbled Salamanders, and spotted Salamanders indicate a complex density-dependent set of interactions among larvae involving both competition and predation, including cannibalism (Cortwright, 1988; Brodman, 1996, 1999b). The effect on Jefferson Salamander population dynamics of interactions with unisexual Ambystoma populations is unknown and may be important with regards to the conservation and status of this species.
Age/Size at Reproductive Maturity - Juveniles mature in 2–3 yr (Bishop, 1941a; P.K. Williams, 1973) and at SVLs of 62–68 mm for males and 76–78 mm for females (Uzzell, 1967a; P.K. Williams, 1973).
Longevity - Jefferson Salamanders were not included among the species that have captive longevity records (Bowler, 1977); no studies using skeletochronology have been conducted. Mark-recapture studies indicate that 10–18% of marked adults survived for 3 yr (Collins, 1965; P.K. Williams, 1973).
Feeding Behavior - Adults and juveniles are thought to feed on earthworms and other soil invertebrates (Downs, 1989b; Petranka, 1998).
Predators - During breeding migrations, adults are vulnerable to predation from mammals such as striped skunks (Mephitis mephitis) and raccoons (Procyon lotor); shrews will feed on adults during other times of the year (Petranka, 1998). Petranka (1998) also suggests owls and woodland snakes will feed on adults and juveniles.
Anti-Predator Mechanisms - Adults elevate the pelvis and tail, undulate the tail and coil the body (Brodie, 1977), and will respond to tongue-flicks from snakes (Dodd, 1977b; Ducey and Brodie, 1983; Brodie, 1989). The dorsal surface of the tail contains glands that produce a noxious, adhesive secretion. In tiger Salamanders, the noxious component of this secretion has neurotoxic effects (Hamning et al., 2000). Larvae seek refuge and reduce activity when in the presence of large tiger Salamander larvae (Brodman and Jaskula, 2002) and adult eastern newts (personal observations).
Diseases - Unknown.
Parasites - Unknown.
Conservation - The main threats to Jefferson Salamanders are habitat destruction and acidification of breeding ponds. They need undisturbed well-drained upland forest sites (Downs, 1989; Petranka, 1998) that are within 200–250 m of seasonal, semi-permanent, and fish-free permanent wetlands (Bishop, 1941; Douglas and Monroe, 1981; Semlitsch, 1998) that are not acidic (Sadinski and Dunson, 1992; Rowe and Dunson, 1993). Because unisexual Ambystoma hybrids often outnumber Jefferson Salamanders when they are syntopic (Uzzell, 1964; Nyman et al., 1988; Bogart and Klemens, 1997; R.B., unpublished data), their interactions, and the conservation implications of these interactions, need to be studied.
References
- Altig, Ronald & McDiarmid, Roy W. 2015. Handbook of Larval Amphibians of the United States and Canada. Cornell University Press, Ithaca, NY. 341 pages.
- AmphibiaWeb. 2020. University of California, Berkeley, CA, USA.
- Bishop, S.C., 1941, The salamanders of New York, New York State Mus. Bull., Vol. 324, pg. 1-365
- Bishop, S.C., 1947, Handbook of Salamanders, 555 pgs., Comstock Publ. Co., Ithaca, N.Y
- Brodie, E.D., Jr., S. Gibson, 1969, Defensive behavior and skin glands of the northwestern salamander, Ambystoma gracile, Herpetologica, Vol. 25, pg. 187-194
- Kumpf, K.F., Yeaton, S.C., 1932, Observation on the courtship behavior of Ambystoma jeffersonianum, Am. Mus. Novit., Num. 546, 7 pgs.
- Minton, S.A., 1972, Amphibians and Reptiles of Indiana, Indiana Academy of Science Monograph, Vol. 3, 346 pgs., Indiana Academy of Science, Indianapolis
- Rand, A.S., 1954, Defense display in the salamander Ambystoma jeffersonianum, Copeia, Vol. 1954, pg. 223-224
- Stille, W.T., 1954, Eggs of the salamander Ambystoma jeffersonianum in the Chicago area, Copeia, Vol. 1954, pg. 300
- Thompson, E.L., Gates, J.E., Taylor, G.J., 1980, Distribution and breeding habitat selection of the Jefferson salamander, Ambystoma jeffersonianum, in Maryland, J. Herpetol., Vol. 14, pg. 113-120
- Uzzell, T.M., Jr., 1964, Relations of the diploid and triploid species of the Ambysoma jeffersonianum complex (Amphibia, Caudata), Copeia, Vol. 1964, pg. 257-300
- Wacasey, J.W., 1961, An ecological study of two sympatric species of salamanders, Ambystoma maculatum and Ambystoma jeffersonianum, in southern Michigan, Ph.D. Diss., Michigan State Univ., East Lansing, UNPB
- Wilson, R.E., 1976, An ecological study of Ambystoma maculatum and Ambystoma jeffersonianum, Ph.D. Diss., Cornell Univ., Ithaca, N.Y., UNPB
- Bishop, S.C., 1943, Handbook of Salamanders, 555 pgs., Comstock Publ. Co., New York, NY
- Martof, B.S., Palmer, W.M., Bailey, J.R., Harrison, III J.R., 1980, Amphibians and Reptiles of the Carolinas and Virginia, 264 pgs., UNC Press, Chapel Hill, NC
- Anderson, J.D., Graham, R.E., 1967, Vertical migration and stratification of larval Ambystoma, Copeia, Vol. 1967, pg. 371-374
- Uzzell, T.M., Jr., 1964, Relations of the diploid and triploid species of the Ambystoma jeffersonianum complex (Amphibia, Caudata), Copeia, Vol. 1964, pg. 257-300
- Ambystoma jeffersonianum (temp. title), Committee on rare and endangered reptiles of Maryland
Photos:
*Click on a thumbnail for a larger version.
Verified County/City Occurrence
Alleghany
Augusta
Bath
Botetourt
Clarke
Craig
Fauquier
Frederick
Giles
Grayson
Highland
Loudoun
Madison
Montgomery
Page
Pulaski
Rappahannock
Rockbridge
Rockingham
Scott
Shenandoah
Warren
Wise
CITIES
Verified in 23 counties and 0 cities.
U.S. Range